
33. Magnesium Iodide (First Update)
Huang, Y.-H.; Lu, H.-H.*
Encyclopedia of Reagents for Organic Synthesis; Submitted (Invited).
32. Concise Total Synthesis of (+)-Shearilicine: A Machine Learning-Assisted Strategy for Ligand Optimization of an Enantioselective Palladium-Catalyzed α-Arylation
Ni, F.-Q.#; Wang, Z.#; Zhang, B.-F.; Li, Z.; Zhang, P.*; Lu, H.-H.*
J. Am. Chem. Soc. In Revision.

31. Natural Product Synthesis Facilitated by the Deuterium Isotopie Effects (DIE)
Chen, X.#; Zhu, Y.#; Suleman, M.; Murmu, R.; Lu, H.-H.*; Chen, Z.*

30. Enantioselective Divergent Total Syntheses of Cycloaurenones and Dysiherbols
Huang, Y.-H.; Gu, Q.-X.; Chao, Q.-C.; Xiao, H.-Z.; Lu, H.-H.*
Angew. Chem. Int. Ed. 2025, 64, e202507638; DOI: 10.1002/anie.202507638 (For preprint, see: ChemRxiv).
- The first total syntheses of cycloaurenones
- Featuring unprecedented desymmetric reactions of a common cyclohexadienone intermediate
- A complementary regioselective quinone alkoxylation protocol to previous methods

- The full story of our synthesis of salimabromide
- A new application of kinetic isotope effects in natural product synthesis

28. Total Synthesis of (-)-Daphenylline by Gold-Catalyzed Intramolecular Dearomatization Reaction of Methoxynaphthalene
Cao, M.-Y.; Gu, Q.-X.; Long, J.; Fang, X.; Lu, H.-H.*
Adv. Synth. Catal. 2024, 366, 4194–4198; DOI: 10.1002/adsc.202400699.
- Invited for a special issue of "Dearomatization" (2024年第三季度“Wiley威立中国高贡献作者”)
- A new concise synthesis of (-)-daphenylline by means of a gold-catalyzed dearomatization reaction of β-methoxynaphthalene
- a new organocatalytic asymmetric Mukaiyama–Michael reaction

27. Asymmetric Hydrogenation of Tetrasubstituted β-Phosphorylated α,β-Unsaturated Esters Catalyzed by Nickel Complexes of Chiral Phosphinooxazolines
Peng, Z.; Gu, Q.-X.; Zhu, Y.; Lu, H.-H.*
Cell Rep. Phys. Sci. 2024, 5, 102261; DOI: 10.1016/j.xcrp.2024.102261.
- The first asymmetric hydrogenation of tetrasubstituted olefins by chiral Ni(II)-PHOX catalysts
- A new class of chiral bisphosphine ligands was developed

- Invited for a special issue of "Emerging Investigators in 2024"
- The first catalytic asymmetric total synthesis
- Strategic use of Rh-catalyzed enantioselective hydrogenation of a tetrahydrodiebenzo[b,d]furan-type tetrasubstituted olefin and C(sp3)-H oxidations

- A powerful general platform for cyclolignan synthesis with ideal modularity and diversity
- Strategic use of Rh-catalyzed enantioselective hydrogenation of a new class of all-carbon tetrasubstituted olefins and C(sp3)-H functionalizations
- Structural revision of a number of cyclolignans
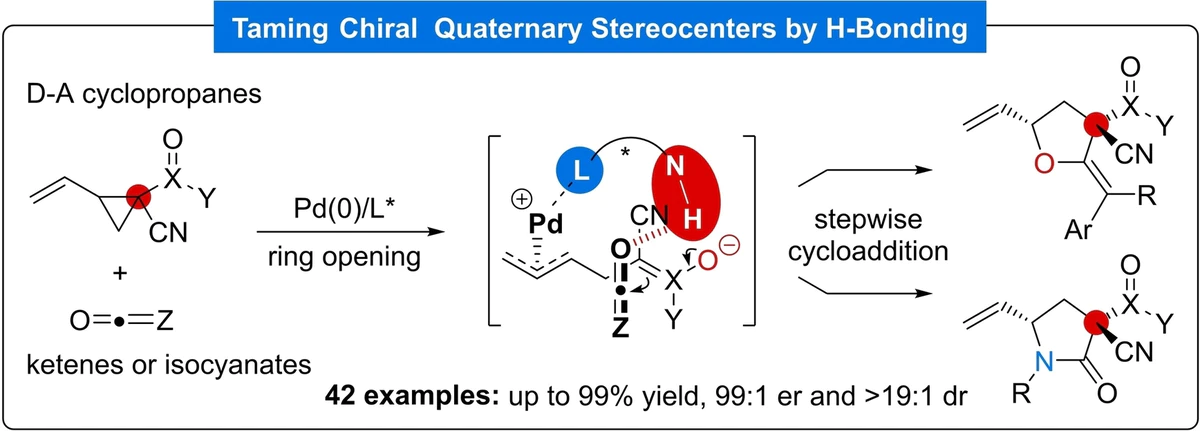
24. Taming Chiral Quaternary Stereocenters via Remote H-Bonding Stereoinduction in Palladium-Catalyzed (3+2) Cycloadditions
Xiao, Y.-Q.; Li, M.-M.; Zhou, Z.-X.; Li, Y.-J. Cao, M.-Y.; Liu, X.-P.; Lu, H.-H.; Rao, L.*; Lu, L.-Q.*; Beauchemin, A. M.; Xiao, W.-J.
Angew. Chem. Int. Ed. 2022, 62, e202212444.
- A new application of our Trost-type ligands (Thanks Liang-Qiu!)
- The first palladium-catalyzed highly diastereo- and enantioselective (3+2) cycloaddition of vinyl cyclopropanes bearing two different electron-withdrawing groups
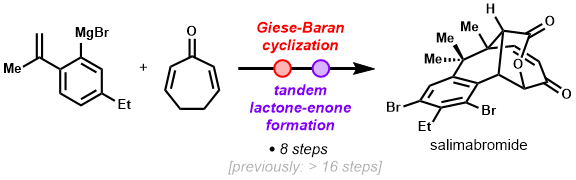
- Simutaneous construction of the unique benzo-fused [4.3.1] framework and the vicinal quaternary carboncenters via a HAT-mediated cyclization (Giese-Baran reaction)
- A novel tandem oxidative reaction was developed to form the butyrolactone and enone moieties concurrently
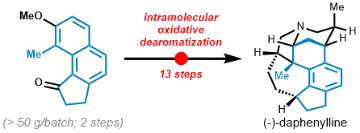
- The first oxidative dearomatization reaction of naphthols for the synthesis of fused rings
- An unprecedented remote acid-directed Mukaiyama-Michael reaction
- A strategic tandem reductive amination/amidation double cyclization reaction
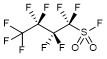
21. Nonafluorobutanesulfonyl Fluoride (Second Update)
Lu, H.-H.*
Encyclopedia of Reagents for Organic Synthesis 2022; DOI: 10.1002/047084289X.rn061.pub3 (Invited).
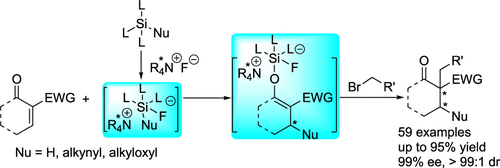
- Vicinal carbon stereocenters constructed with excellent selectivities (up to 99% ee, >99:1 dr)
- Penta-coordinated silicates are crucial intermediates
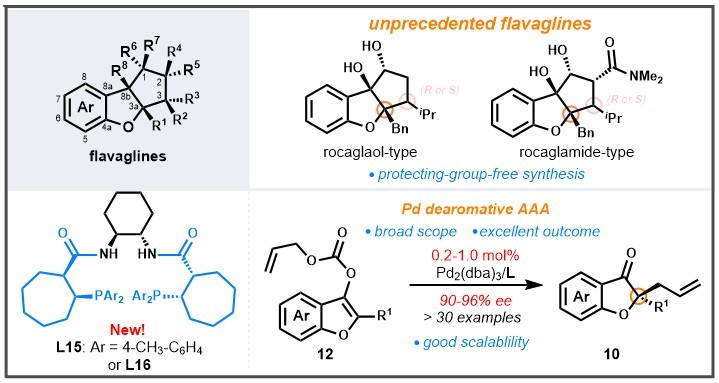
19. Enantioselective Palladium-Catalyzed Decarboxylative Dearomative Asymmetric Allylic Alkylation of Benzofurans: Diversity-Oriented Synthesis of Flavaglines
Lu, H.-H.*; Cao, M.-Y.
Synlett (Synpacts) 2021, 1981–1986; DOI: 10.1055/a-1650-4266 (Invited).
18. Optically Active Flavaglines-Inspired Molecules by a Palladium-Catalyzed Decarboxylative Dearomative Asymmetric Allylic Alkylation
Cao, M.-Y.; Ma, B.-J.; Lao, Z.-Q.; Wang, H.; Wang, J.; Liu, J.; Xing, K.; Huang, Y.-H.; Gan, K.-J.; Gao, W.; Wang, H.; Hong, X.; Lu, H.-H.*
J. Am. Chem. Soc. 2020, 142, 12039–12045; DOI: 10.1021/jacs.0c05113.
- A new class of Trost-type ligands bearing a chiral cycloalkane framwork
- An unprecedented Pd-catalyzed dearomative AAA (> 30 examples, up to 96% ee)
- A new blueprint for flavagline-based drug development by a DOS design
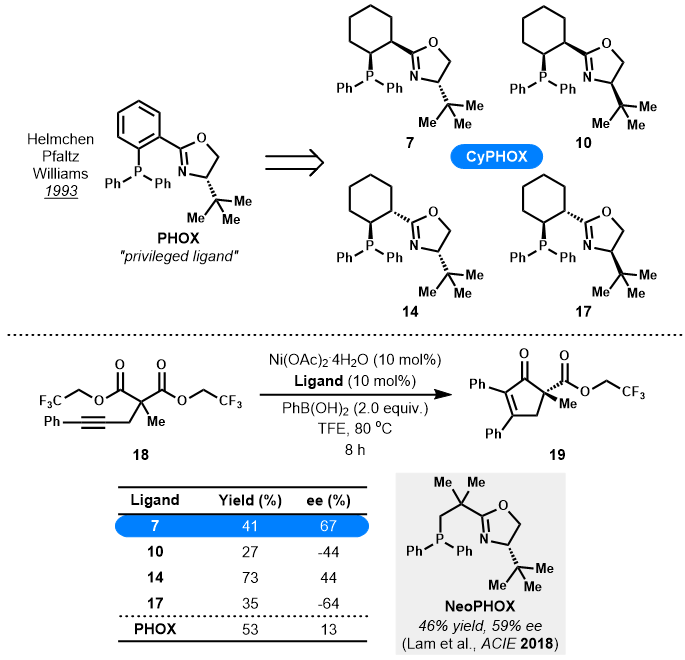
- Invited for the Ryan Shenvi Award Issue
- Four newly designed CyPHOX ligands with chiral cyclohexane scaffold replacing the previous planar aryl group of PHOX ligands, were efficiently prepared.
- Preliminary tests with these CyPHOX ligands showed that they exhibited better performance (44-67% ee) than the privileged PHOX ligand (13% ee) in Lam's nickel-catalyzed desymmetrizing arylative cyclization
16. Witkin, J. M.*; Shenvi, R. A.; Li, X.; Gleason, S. D.; Weiss, J.; Morrow, D.; Catow, J. T.; Wakulchik, M.; Ohtawa, M.; Lu, H.-H.; Martinez, M. D.; Schkeryantz, J. M.; Carpenter, T. S.; Lightstone, F. C.; Cerne, R. Pharmacological characterization of the neurotrophic sesquiterpene jiadifenolide reveals a non-convulsant signature and potential for progression in neurodegenerative disease studies. Biochem. Pharm. 2018, 155, 61–70; DOI: 10.1016/j.bcp.2018.06.022.
15. Lu, H.-H.#; Pronin, S.#; Antonova-Koch, Y.; Meister, S.; Winzeler, E. A.; Shenvi, R. A.* Synthesis of (+)-7,20-Diisocyanoadociane and Liver Stage Antiplas-modial Activity of the ICT Class. J. Am. Chem. Soc. 2016, 138, 7268–7271; DOI: 10.1021/jacs.6b03899.
14. Lu, H.-H.; Martinez, M. D.; Shenvi, R. A.* An Eight-step, Gram-Scale Synthesis of (–)-Jiadifenolide. Nat. Chem. 2015, 7, 604–607; DOI:10.1038/nchem.2283.
Highlighted in Chemistry World, July 27 2015;
Highlighted by E. M. Carreira and M. Westph, Synfacts 2015, 11, 089;
#1 most read article, June-July 2015.
13. Lu, H.-H.; Hinkelmann, B.; Tautz, T.; Li, J.; Franke, R.; Sasse, F.; Kalesse, M.* Paleo-Soraphens: Chemical Total Syntheses and Biological Studies. Org. Biomol. Chem. 2015, 13, 8029–8036; DOI: 10.1039/C5OB01249J.
12. Kalesse, M.*; Cordes, M.; Symkenberg, G.; Lu, H.-H. The Vinylogous Mukaiyama Aldol Reaction (VMAR) in Natural Product Synthesis. Nat. Prod. Rep. 2014, 31, 563–594; DOI: 10.1039/C3NP70102F.
11. Lu, H.-H.; Raja, A.; Franke, R.; Landsberg, D.; Sasse, F.; Kalesse, M.* Syntheses and Biological Evaluation of Paleo-Soraphens. Angew. Chem. Int. Ed. 2013, 52, 13549–13552; DOI: 10.1002/anie.201305331.
10. Duan, S.-W.; Lu, H.-H.; Zhang, F.-G.; Xuan, J.; Chen, J.-R.; Xiao, W.-J.* Organocatalytic Conjugate Additions of Acetylacetone to 3-Ylideneoxindoles: A Direct Access to Highly Enantioenriched Oxindole Derivatives. Synthesis 2011, 1847–1852; DOI: 10.1055/s-0030-1260463.
9. Lu, H.-H.; Tan, F.; Xiao, W.-J.* Enantioselective Organocatalytic Friedel-Crafts Alkylations. Curr. Org. Chem. 2011, 15, 4022–4045; DOI: 10.2174/138527211798109196. (Invited Review)
8. Zhang, F.-G.; Yang, Q.-Q.; Xuan, J.; Lu, H.-H.; Duan, S.-W.; Chen, J.-R.; Xiao, W.-J.* Enantioselective Conjugate Addition of Oximes to Trisubstituted β-Nitroacrylates: An Organocatalytic Approach to β2,2-Amino Acid Derivatives. Org. Lett. 2010, 12, 5636–5639; DOI: 10.1021/ol102580n.
7. Lu, H.-H.; Liu, H.; Wu, W.; Wang, X.-F.; Lu, L.-Q.; Xiao, W.-J.* Catalytic Asymmetric Intramolecular Hydroarylations of ω-Aryloxy- and Arylamino Tethered α,β-Unsaturated Aldehydes. Chem. Eur. J. 2009, 15, 2742–2746; DOI: 10.1002/chem.200802722.
6. Lu, H.-H.; Meng, X.-G.; Zhang, F.-G.; Duan, S.-W.; Xiao, W.-J.* Enantioselective Michael Reactions of β,β-Disubstituted Nitroalkenes: A New Approach to β2,2-Amino Acids with Hetero-Quaternary Stereocenters. Org. Lett. 2009, 11, 3946–3949; DOI: 10.1021/ol901572x.
5. Lu, H.-H.; Wang, X.-F.; Yao, C.-J.; Zhang, J.-M.; Wu, H.; Xiao, W.-J.* Highly Enantioselective Organocatalytic Michael Addition of Nitroalkanes to 4-oxo-Enoates. Chem. Commun. 2009, 4251–4253; DOI: 10.1039/B905033G.
4. Chen, J.-R.; Lai, Y.-Y.; Lu, H.-H.; Wang, X.-F.; Xiao, W.-J.* Highly Enantioselective Desymmetrization of meso- and Prochiral Cyclic Ketones via Organocatalytic Michael Reaction. Tetrahedron 2009, 65, 9238–9243; DOI: 10.1016/j.tet.2009.09.005.
3. Dong, H.-M.; Lu, H.-H.; Lu, L.-Q.; Chen, C.-B.; Xiao, W.-J.* Asymmetric Friedel-Crafts Alkylations of Indoles with Ethyl Glyoxylate Catalyzed by (S)-BINOL-Ti (IV) Complex: Direct Access to Enantiomerically Enriched 3-Indolyl-hydroxyacetates. Adv. Synth. Catal. 2007, 349, 1597–1603; DOI: 10.1002/adsc.200600495.
2. Cao, Y.-J.; Lu, H.-H.; Lai, Y.-Y.; Lu, L.-Q.; Xiao, W.-J.* An Effective Bifunctional Thiourea Catalyst for Highly Enantio- and Diastereoselective Michael Addition of Cyclohexanone to Nitroolefins. Synthesis 2006, 3795–3800; DOI: 10.1055/s-2006-950339.
1. Chen, J.-R.; Lu, H.-H.; Li, X.-Y.; Cheng, L.; Wan, J.; Xiao, W.-J.* Readily Tunable and Bifunctional L-Prolinamide Derivatives: Design and Application in the Direct Enantioselective Aldol Reactions. Org. Lett. 2005, 7, 4543–4545; DOI: 10.1021/ol0520323.
#These authors contributed equally.
Copyright 2017 The Lu Research Lab. All Rights Reserved.














